| property | value |
|---|---|
| Common names | Diphenidine |
| Systematic name | (±)-1-(1,2-Diphenylethyl)piperidine |
| Psychoactive class | Dissociative |
| Chemical class | Diarylethylamine |
diphenidine is a lesser-known novel dissociative substance of the diarylethylamine class that produces dissociative and hallucinogenic effects when administered. it is structurally related to methoxphenidine and ephenidine.
the synthesis of diphenidine was first reported in 1924. shortly after the 2013 uk arylcyclohexylamine ban, diphenidine and the related compound methoxphenidine became available on the grey market. in 2014, there were two cases of diphenidine being sold in combination with synthetic cannabinoids in japanese herbal incense blends, one of which was implicated in a fatal overdose.
diphenidine is classified as an nmda receptor antagonist. members of this class induce a state known as “dissociative anesthesia” and are used in both medical and recreational contexts. these include arylcyclohexylamines like ketamine and phencyclidine (pcp), as well as dextromethorphan (dxm).
anecdotal reports describe high doses of diphenidine producing “bizarre somatosensory phenomena and transient anterograde amnesia.”
very little data exists about the pharmacological properties, metabolism, and toxicity of diphenidine in humans, and it has an extremely limited history of human usage. many reports suggest that it may pose different risks than traditional dissociatives. it is highly advised to use harm reduction practices if using this substance.
history and culture
the synthesis of diphenidine was first reported in 1924. it employed a nitrile displacement reaction analogous to the one that would later be used to discover phencyclidine in 1956. shortly after the 2013 uk ban on arylcyclohexylamines, diphenidine and the related compound methoxphenidine became available on the grey market.
in 2014, there were two cases of diphenidine being sold in combination with synthetic cannabinoids in japanese herbal incense blends. one herbal incense blend was found to contain diphenidine and 5-fluoro-ab-pinaca at concentrations of 289 mg/g and 55.5 mg/g, respectively. another product containing ab-chminaca, 5f-amb, and diphenidine was implicated in a fatal overdose.
chemistry
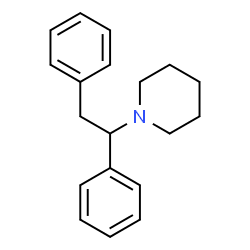
diphenidine is a molecule of the diarylethylamine class. it contains a substituted phenethylamine skeleton with an additional phenyl ring bound to rα. the terminal amino group of the phenethylamine chain is incorporated into a piperidine ring. hence, diphenidine belongs to the piperidine dissociative class of compounds. diphenidine is structurally analogous to mxp, lacking a 2-methoxy substitution on one of its phenyl rings.
pharmacology
diphenidine acts as an nmda receptor antagonist. nmda receptors allow for electrical signals to pass between neurons in the brain and spinal column; for the signals to pass, the receptor must be open. dissociatives close the nmda receptors by blocking them. this disconnection of neurons leads to loss of feeling, difficulty moving, and eventually the famous “hole”.
although vendors of diphenidine have stated the compound acts as a dopamine–reuptake inhibitor and a serotonin reuptake inhibitor with µ-opioid affinity and typical dissociative effects, to date diphenidine has not been screened for affinity at the dopamine transporter. if this is indeed the case, however, it provides an explanation for its euphoric and often stimulating effects.
diphenidine and related diarylethylamines have been studied in vitro as treatments for neurotoxic injury. diphenidine may be a stronger nmda receptor antagonist for neurogenesis, neurological repair and neuroprotection than other more common nmda receptor antagonistic dissociatives such as ketamine, dextromethorphan, pcp analogs, iboga and methoxetamine.


MishaY. –